Diabetes mellitus, a chronic metabolic disorder characterized by elevated blood glucose levels, encompasses two primary forms: type 1 and type 2. While both share the hallmark of hyperglycemia, their underlying causes, disease mechanisms, and relationship with obesity diverge significantly. Understanding these distinctions is crucial for effective management and treatment strategies.
Type 1 Diabetes: An Autoimmune Assault
Type 1 diabetes, previously known as insulin-dependent or juvenile diabetes, arises from an autoimmune reaction. In this condition, the body’s immune system mistakenly identifies and attacks the insulin-producing beta cells in the pancreas. This relentless destruction leads to a severe or absolute deficiency of insulin, a hormone essential for glucose uptake by cells for energy.
The exact triggers for this autoimmune assault remain elusive, but genetic predisposition and environmental factors, such as viral infections, are believed to play a role. Unlike type 2 diabetes, type 1 is not directly caused by lifestyle factors like obesity. Individuals with type 1 diabetes require lifelong insulin therapy, typically through injections or an insulin pump, to regulate their blood glucose levels and survive.
While obesity is not the primary cause of type 1 diabetes, it can still present a challenge for individuals with the condition. Obesity can lead to insulin resistance, meaning the body’s cells become less responsive to the effects of insulin. This can complicate blood glucose management in type 1 diabetes, potentially requiring higher insulin doses and increasing the risk of hypoglycemia (low blood sugar) if not carefully balanced with diet and exercise. Recent research indicates that the prevalence of overweight and obesity in adults with type 1 diabetes is nearly the same as in the general population, highlighting the importance of addressing weight management in this population.
Type 2 Diabetes: A Complex interplay of Insulin Resistance and Deficiency
Type 2 diabetes, formerly known as non-insulin-dependent or adult-onset diabetes, is a more complex condition characterized by a combination of insulin resistance and relative insulin deficiency. In insulin resistance, the body’s cells, particularly muscle, fat, and liver cells, become less sensitive to the action of insulin. Initially, the pancreas tries to compensate by producing more insulin, but over time, it may not be able to keep up with the increased demand, leading to elevated blood glucose levels.
Obesity is a major risk factor for developing type 2 diabetes. Excess body weight, particularly abdominal fat, is strongly linked to increased insulin resistance. Adipose tissue releases various hormones and fatty acids that can interfere with insulin signaling pathways. Furthermore, obesity can contribute to chronic low-grade inflammation, which also impairs insulin sensitivity and damages pancreatic beta cells over time.
The progression to type 2 diabetes is often gradual. Many individuals may have prediabetes, a condition where blood glucose levels are higher than normal but not yet in the diabetic range. Lifestyle modifications, including weight loss through diet and exercise, can often prevent or delay the progression from prediabetes to type 2 diabetes.
While not all individuals with type 2 diabetes are obese, a significant proportion are overweight or obese at the time of diagnosis. In these cases, weight management becomes a cornerstone of treatment. Losing even a modest amount of weight can improve insulin sensitivity, lower blood glucose levels, and reduce the need for medications. However, type 2 diabetes can also develop in individuals who are not overweight, often due to a combination of genetic predisposition and other factors affecting insulin secretion.
Obesity: A Common Thread with Distinct Implications
Obesity plays a contrasting role in type 1 and type 2 diabetes. In type 1, it is not a cause but a complicating factor that can affect blood glucose control due to increased insulin resistance. In type 2, obesity is a primary risk factor, directly contributing to the development of insulin resistance and eventually relative insulin deficiency.
The mechanisms by which obesity contributes to insulin resistance in type 2 diabetes are multifaceted:
- Increased Free Fatty Acids: Excess adipose tissue leads to elevated levels of free fatty acids in the bloodstream. These fatty acids can impair insulin signaling in muscle and liver cells.
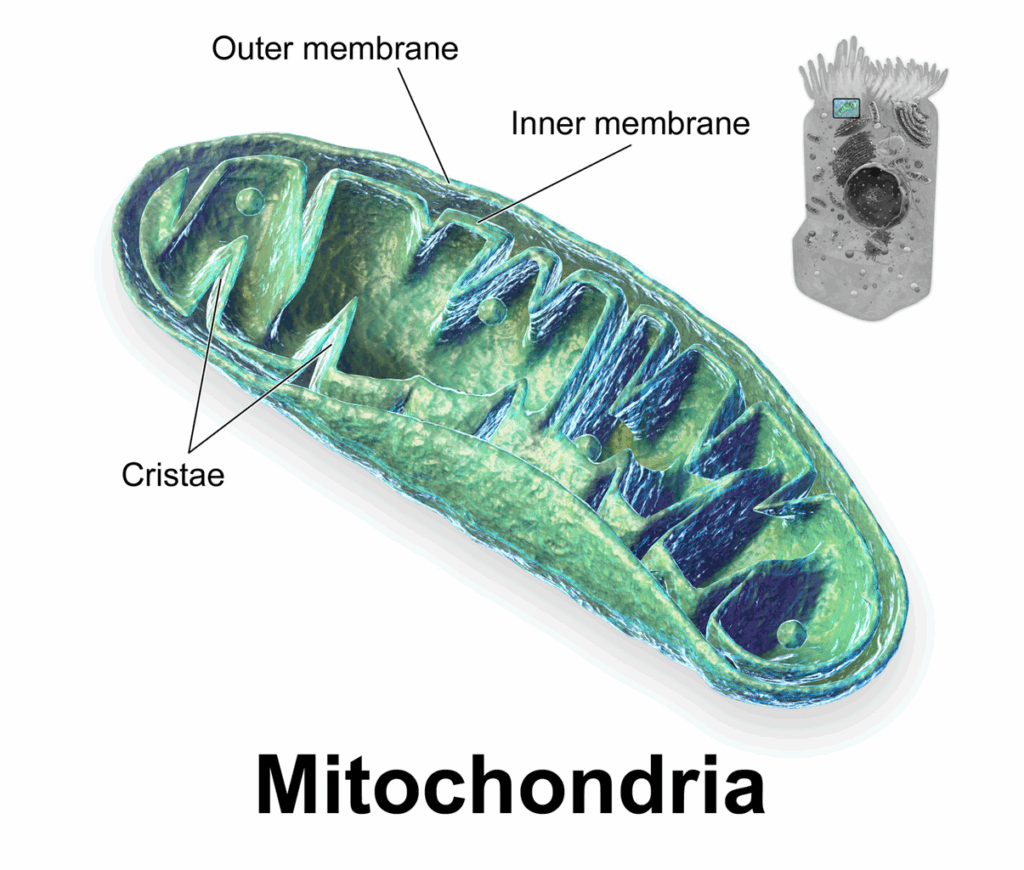
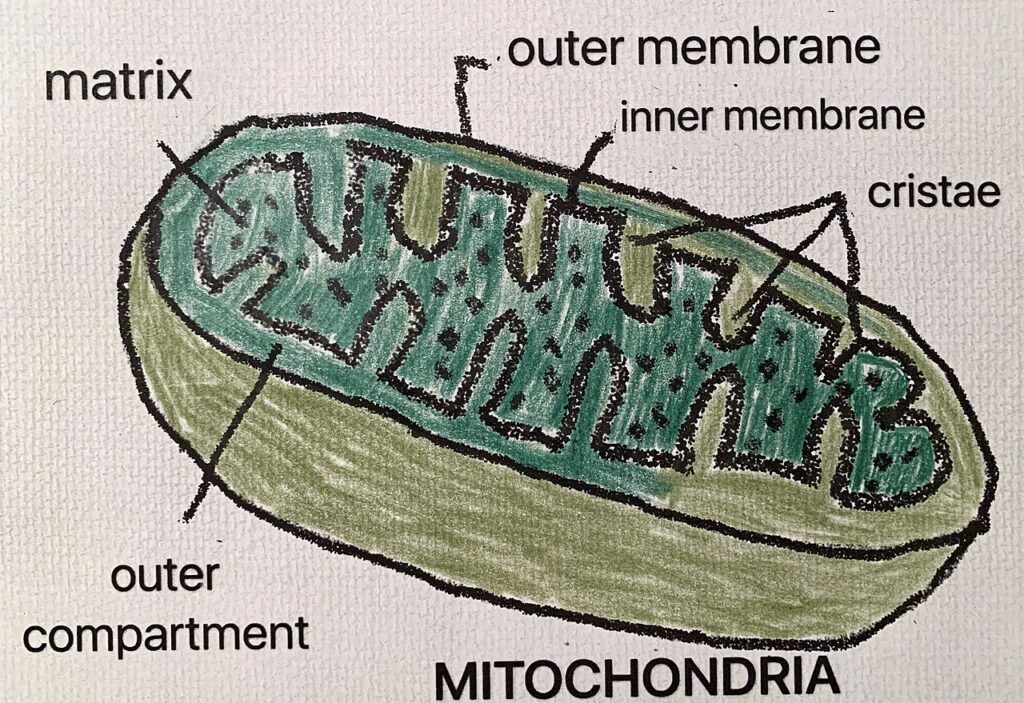
- Adipokine Dysregulation: Adipose tissue secretes various hormones called adipokines. In obesity, the secretion of beneficial adipokines like adiponectin decreases, while the secretion of pro-inflammatory adipokines like TNF-alpha and interleukin-6 increases. This imbalance contributes to insulin resistance and beta-cell dysfunction.
- Chronic Inflammation: Obesity is associated with chronic low-grade inflammation. Inflammatory cytokines can interfere with insulin signaling and damage pancreatic beta cells.
- Ectopic Fat Deposition: Excess fat can accumulate in organs like the liver and muscles, further impairing their ability to respond to insulin.
Management Strategies: Tailored Approaches
The fundamental difference in the underlying causes of type 1 and type 2 diabetes necessitates distinct management strategies, even when obesity is present.
- Type 1 Diabetes and Obesity: The primary treatment remains insulin therapy. However, weight management through a balanced diet and regular exercise is crucial to improve insulin sensitivity and overall health. Careful monitoring of blood glucose is essential to adjust insulin doses based on dietary intake and physical activity, especially considering the increased risk of hypoglycemia with weight loss efforts.
- Type 2 Diabetes and Obesity: Lifestyle interventions, including weight loss, a healthy eating plan, and regular physical activity, are often the first line of treatment. These measures can significantly improve insulin sensitivity and blood glucose control. Medications that enhance insulin sensitivity, increase insulin secretion, or reduce glucose production may also be necessary. In some cases, bariatric surgery can be aConsideration for individuals with severe obesity and type 2 diabetes.
Conclusion: Distinct Entities, Shared Risks
Type 1 and type 2 diabetes are distinct conditions with different origins. Type 1 diabetes is an autoimmune disease leading to absolute insulin deficiency, while type 2 diabetes is characterized by insulin resistance and relative insulin deficiency, with obesity being a major contributing factor. While obesity complicates the management of both types of diabetes, its role in the development of type 2 diabetes is far more direct and significant. Recognizing these differences is essential for guiding appropriate prevention, management, and treatment strategies to improve the lives of individuals living with these conditions.
Click Here to Buy Mitolyn Supplement to Help Fight Against Obesity!